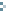- ''::ANNEX I::'' /> />''__::HAZARD ANALYSIS AND CRITICAL CONTROL POINTS -HACCP- PRINCIPLES AND GUIDELINES FOR THEIR APPLICATION::__''
+ ^::__~~#4B0082:ANNEX I - HAZARD ANALYSIS AND CRITICAL CONTROL POINTS -HACCP- PRINCIPLES AND GUIDELINES FOR THEIR APPLICATION~~__::^
::__Introduction__::
establish and implement a monitoring system at each critical point.
+
+ __3. CRITICAL LIMITS AT CRITICAL CONTROL POINTS__
+
+ Each control measure associated with a critical control point should give rise to the specification of critical limits.
+
+ Critical limits correspond to the extreme values acceptable with regard to product safety. They separate acceptability from unacceptability. They are set for observable or measurable parameters which can demonstrate that the critical point is under control. They should be based on substantiated evidence that the chosen values will result in process control.
+
+ Examples of such parameters include temperature, time, pH, moisture content, additive, preservative or salt level, sensory parameters such as visual appearance or texture, etc.
+ In some cases, to reduce the risk of exceeding a critical limit due to process variations, it may be necessary to specify more stringent levels (i.e. target levels) to assure that critical limits are observed.
+
+ Critical limits may be derived from a variety of sources. When not taken from regulatory standards or from guides of good hygiene practices, the team should ascertain their validity relative to the control of identified hazards at CCP’s.
+
+ __4. MONITORING PROCEDURES AT CRITICAL CONTROL POINTS__
+
+ An essential part of HACCP is a program of observations or measurements performed at each critical point to ensure compliance with specified critical limits..
+
+ Observations or measurements must be able to detect loss of control at critical points and provide information in time for corrective action to be taken.
+
+ Where possible, process adjustments should be made when monitoring results indicate a trend towards loss of control at a CCP. The adjustments should be taken before a deviation occurs. Data derived from monitoring must be evaluated by a designated person with knowledge and authority to carry out corrective actions when indicated.
+
+ Observations or measurements can be made continuously or intermittently. When observations or measurements are not continuous, it is necessary to establish a frequency of observations or measurements which provides reliable information.
+
+ The program should describe the methods, the frequency of observations or measurements and the recording procedure and identify each critical point:
+ *who is to perform monitoring and checking,
+
+ *when monitoring and checking is performed,
+
+ *how monitoring and checking is performed.
+
+ Records associated with monitoring CCP’s must be signed by the person(s) doing the monitoring and when records are verified by a responsible reviewing official(s) of the company.
+
+ __5. CORRECTIVE ACTIONS __
+
+ For each critical control point corrective actions have to be planned in advance by the HACCP team, so that they can be taken without hesitation when monitoring indicates a deviation from the critical limit.
+
+ Such corrective action should include:
+ *proper identification of the person(s) responsible for the implementation of the corrective action,
+
+ *description of means and action required to correct the observed deviation,
+
+ *action to be taken with regard to products that have been manufactured during the period when the process was out of control,
+
+ *written record of measures taken indicating all relevant information (for example: date, time, type of action, actor and subsequent verification check).
+
+ Monitoring may indicate:
+
+ 2. that preventive measures (checking equipment, checking the person handling the food, checking the efficacy of previous corrective measures, etc.) shall have to be taken if corrective actions for the same procedure have to be taken repeatedly.
+
+ __6. VERIFICATION PROCEDURES__
+
+ 6.1. The HACCP team should specify the methods and procedures to be used for determining if the HACCP is working correctly. Methods for verification may include in particular random sampling and analysis, reinforced analysis or tests at selected critical points, intensified analysis of intermediate or final products, surveys on actual condition during storage, distribution and sale and on actual use of the product.
+
+ The frequency of verification should be sufficient to confirm that HACCP is working effectively. The frequency of verification shall depend on the characteristics of the business (output, number of employees, nature of the food handled), the monitoring frequency, the accuracies of the employees, the number of deviations detected over time and the hazards involved.
+
+ Verification procedures include:
+ *audits of HACCP and its records,
+
+ *inspection of operations,
+
+ *Confirmation that CCP’s are kept under control,
+
+ *validation of critical limits,
+
+ *review of deviations and product dispositions; corrective actions taken with regard to the product.
+
+ The frequency of verification will greatly influence the amount of recheck or recall required in case a deviation exceeding the critical limits has been detected. Verification shall comprise all of the following elements, but not necessarily all at the same time:
+ *check on the correctness of the records and analysis of deviations
+
+ *check on the person monitoring processing, storage and/or transport activities
+
+ *physical check on the process being monitored
+
+ *calibration of instruments used for monitoring.
+
+ Verification should be carried out by someone other than the person who is responsible for performing the monitoring and corrective actions. Where certain verification activities cannot be performed in house, verification should be performed on behalf of the business by external experts or qualified third parties.
+
+ 6.2. Where possible, validation activities should include actions to confirm the efficacy of all elements of the HACCP plan. In case of change, it is necessary to review the system, to ensure that it is (or will be) still valid.
+ Examples of change include:
+
+ *change in raw material or in product, processing conditions (factory layout and environment, process equipment, cleaning and disinfection program),
+
+ *change in packaging, storage or distribution conditions,
+
+ *change in consumer use,
+
+ *receipt of any information on a new hazard associated with the product.
+
+ Where necessary, such a review must result in the amendment of the procedures laid down. The changes should be fully incorporated into the documentation and record-keeping system in order to ensure that accurate up-to-date information is available.
+
+ __7. DOCUMENTATION AND RECORD KEEPING__
+
+ Efficient and accurate record keeping is essential to the application of a HACCP system. HACCP procedures should be documented. Documentation and record keeping should be appropriate to the nature and size of the operation and sufficient to assist the business to verify that the HACCP controls are in place and being maintained. Documents and records should be kept for a sufficient time to allow the competent authority to audit the HACCP system. Expertly developed HACCP guidance materials (e.g. sector-specific HACCP guides) may be utilized as part of the documentation, provided that those materials reflect the specific food operations of the business. Documents should be signed by a responsible reviewing official of the company.
+
+ Documentation examples are:
+ *Hazard analysis;
+
+ *CCP determination;
+
+ *Critical limit determination;
+
+ *Modifications to the HACCP system.
+
+ Record examples are:
+ *CCP monitoring activities;
+
+ *Deviations and associated corrective actions;
+
+ *Verification activities.
+
+ A simple record-keeping system can be effective and easily communicated to employees. It may be integrated into existing operations and may use existing paperwork, such as delivery invoices and checklists to record, for example, product temperatures.
+
+ __8. TRAINING __
+
+ 1. The food business operator shall make sure that all personnel are aware of the hazards identified (if any), the critical points in the production, storage, transport and/or distribution process and the corrective measures, the preventive measures and documentation procedures applicable in his/her business.
+
+ 2. The food industry sectors shall endeavour to prepare information such as. (generic) HACCP guides and training for the food business operators.
+
+ 3. The competent authority shall, when needed, assist in developing similar activities as mentioned in paragraph 2, especially in those sectors, which are poorly organised or are shown to be insufficiently informed.
+
+ Figure 1: Example of a decision tree to identify critical control points (CCP’s). The questions shall be answered in sequence.
+
+ ::{img src="http://www.cytali.org/tiki/show_image.php?id=64" width= height= align= desc= link= }::
+
+ [http://www.cytali.org/tiki/tiki-index.php?page=en32004HACCP|Anterior] - [http://www.cytali.org/tiki/tiki-index.php?page=ena22004HACCP|Siguiente]
|